¿Qué causa los poros dilatados y cómo minimizarlos?
¿Luchas contra los poros dilatados? Aunque pueden no ser tan preocupantes como las cicatrices de acné o las arrugas, los poros visibles pueden hacer que tu piel parezca áspera y desigual. Hasta el mejor base de maquillaje podría fallar en dar un acabado impecable si los poros son demasiado grandes, especialmente en áreas como la nariz, mejillas y frente.
Entonces, ¿qué causa la dilatación de los poros y cómo reducir su apariencia? Exploremos la ciencia detrás de los poros y descubramos tratamientos efectivos para refinar tu piel.
¿Por qué se dilatan los poros?
Los poros dilatados son una preocupación cutánea común, frecuentes en nariz, mejillas, frente y mentón. Aunque no son un problema médico, pueden hacer que la piel luzca áspera e irregular. Esto también dificulta la aplicación de maquillaje, ya que las bases y polvos tienden a acumularse en estos poros. Entender las causas es el primer paso hacia un tratamiento efectivo.

Las 4 principales causas de poros dilatados
1. Exceso de producción sebácea
Las glándulas sebáceas hiperactivas son un factor clave. El exceso de sebo se mezcla con células muertas y obstruye los poros, expandiéndolos. Común en pieles grasas, influenciado por hormonas, estrés y hábitos.
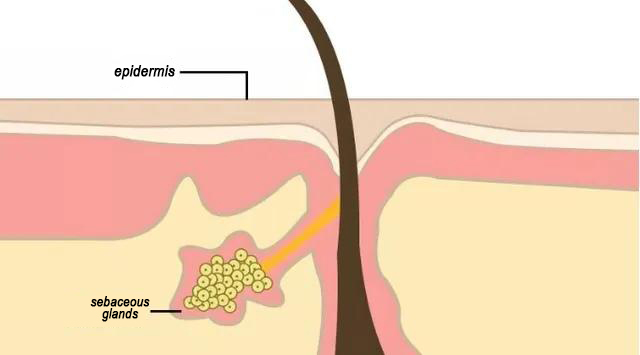
2. Piel deshidratada
Irónicamente, las pieles secas también pueden tener poros dilatados. La falta de hidratación estimula mayor producción de sebo, empeorado por ambientes secos, productos agresivos e hidratación insuficiente.
3. Envejecimiento y daño solar
Con la edad, perdemos colágeno y elasticidad, haciendo los poros más visibles. La exposición prolongada a radiación UV debilita la estructura cutánea, causando poros alargados.

4. Genética y folículos pilosos
Algunos tienen poros grandes por genética. Más folículos pilosos en nariz/mejillas también contribuyen. Contaminantes y células muertas acumuladas empeoran este problema.
Mitos comunes sobre reducir poros
Muchos prueban remedios caseros ineficaces. Desmontemos mitos populares:
1. Hielo o agua fría/caliente
Los poros no tienen músculos para contraerse. Los cambios bruscos de temperatura irritan la piel, causando sensibilidad.

2. Jugo de limón
Su alta acidez altera el pH cutáneo, causando irritación y daño solar.
3. Exfoliantes de azúcar
Los gránulos irregulares causan microdesgarros, debilitando la barrera cutánea.

Métodos comprobados para reducir poros
Soluciones dermatológicas recomendadas:
1. Control sebáceo y limpieza profunda (piel grasa)
Productos clave:
- Retinol: Regula sebo y estimula colágeno
- Niacinamida: Reduce inflamación
- Ácido salicílico y glicólico: Exfolian células muertas
Tratamientos médicos:
- Retinoides tópicos (usar con precaución)
- Inyecciones de Botox para glándulas sebáceas
2. Hidratación (piel seca)
Ingredientes esenciales:
- Ácido hialurónico: Retiene humedad
- Ceramidas: Fortalecen barrera cutánea
- Panthenol: Repara piel seca
Usar mascarilla hidratante 1-2 veces/semana

3. Protección solar y estimulación de colágeno (piel madura)
Recomendaciones:
- Protector solar contra daño UV
- Péptidos para producción de colágeno
- Vitamina C como antioxidante
Tratamientos avanzados:
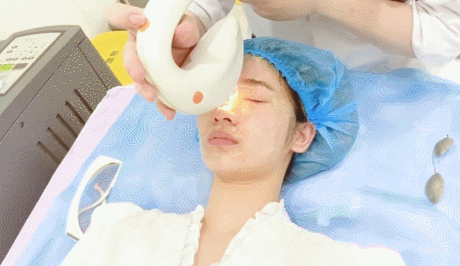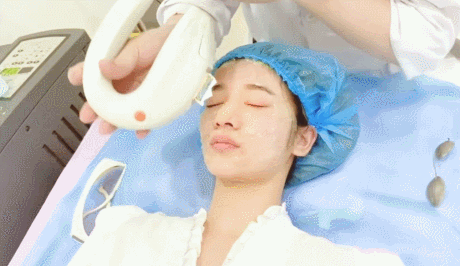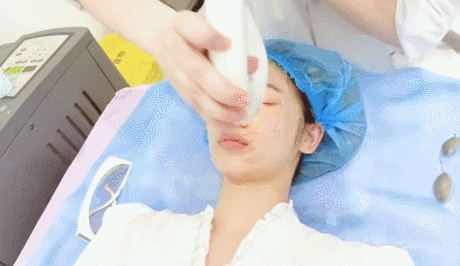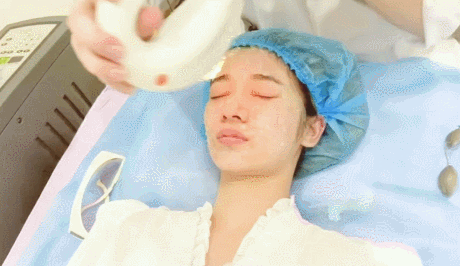
- Microneedling con radiofrecuencia
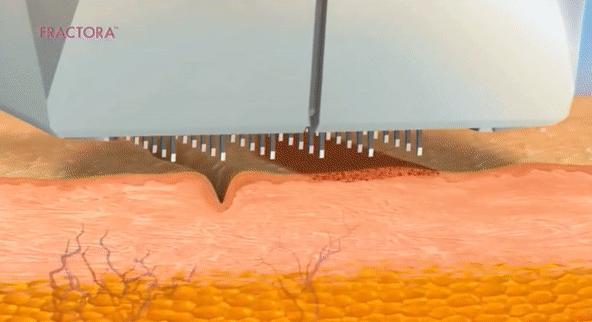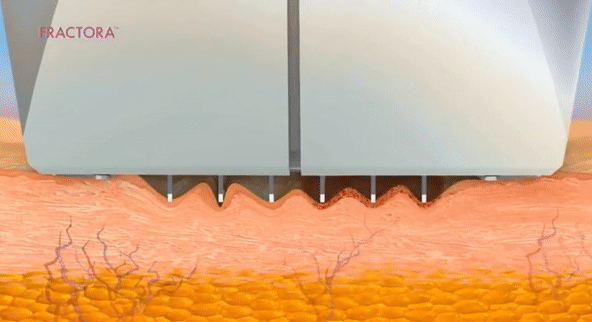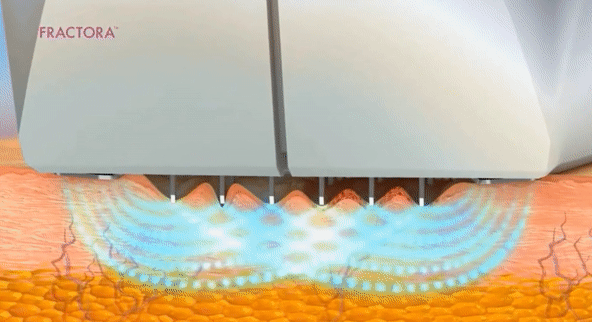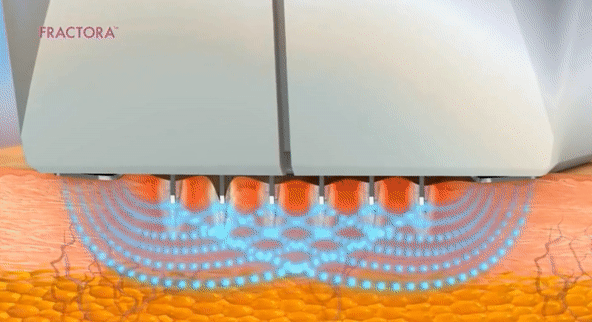
- Láser fraccionado (requiere recuperación)
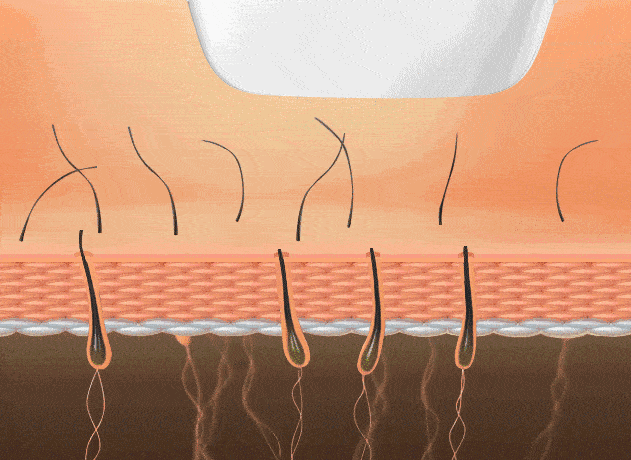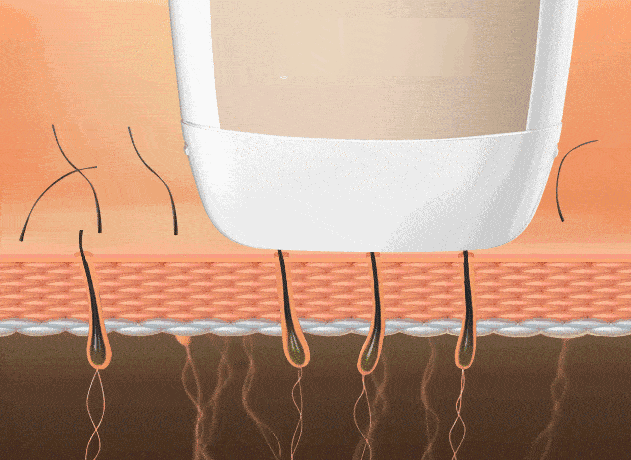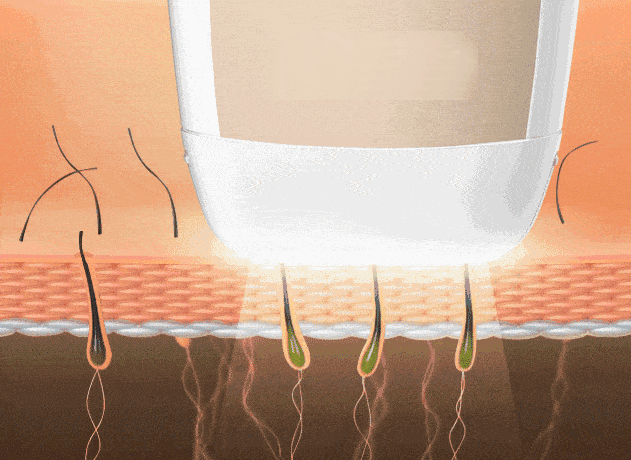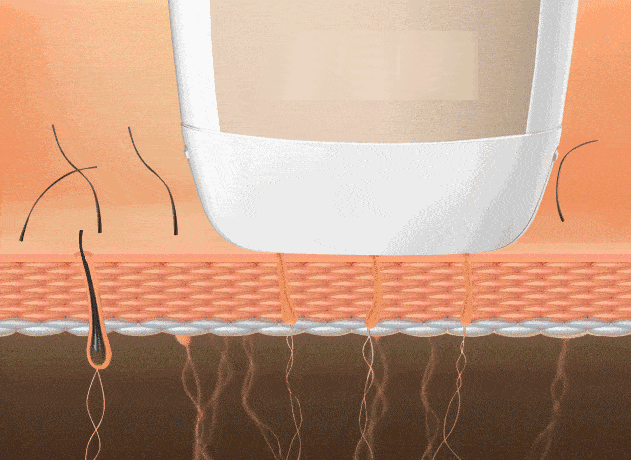
4. Depilación láser (poros foliculares)
Reduce el crecimiento capilar para piel más lisa. La depilación por “punto frío” minimiza molestias.
Reflexión final
Los poros dilatados pueden manejarse con rutinas adecuadas y tratamientos profesionales. Evita remedios caseros ineficaces. Ya sea mediante hidratación, protección solar o procedimientos dermatológicos, cada tipo de piel tiene solución. Entender tus necesidades cutáneas es clave para un cutis refinado y saludable.



















